SCI Publications
2011
J.R. Anderson, B.W. Jones, C.B. Watt, M.V. Shaw, J.-H. Yang, D. DeMill, J.S. Lauritzen, Y. Lin, K.D. Rapp, D. Mastronarde, P. Koshevoy, B. Grimm, T. Tasdizen, R.T. Whitaker, R.E. Marc.
“Exploring the Retinal Connectome,” In Molecular Vision, Vol. 17, pp. 355--379. 2011.
PubMed ID: 21311605
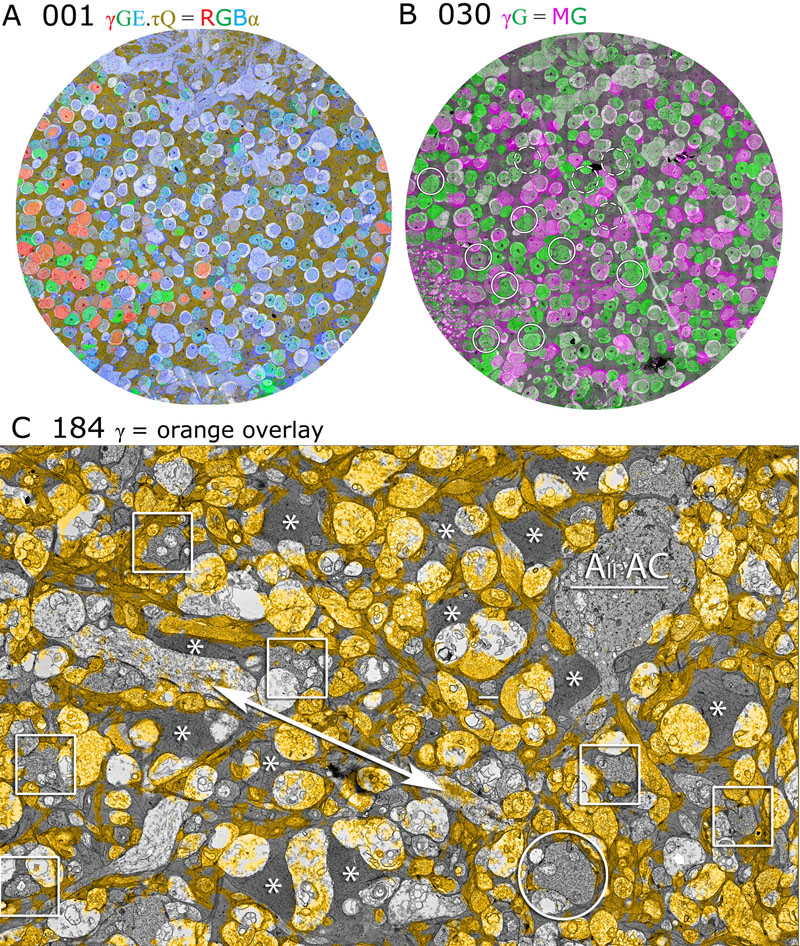
Purpose: A connectome is a comprehensive description of synaptic connectivity for a neural domain. Our goal was to produce a connectome data set for the inner plexiform layer of the mammalian retina. This paper describes our first retinal connectome, validates the method, and provides key initial findings.
Methods: We acquired and assembled a 16.5 terabyte connectome data set RC1 for the rabbit retina at .2 nm resolution using automated transmission electron microscope imaging, automated mosaicking, and automated volume registration. RC1 represents a column of tissue 0.25 mm in diameter, spanning the inner nuclear, inner plexiform, and ganglion cell layers. To enhance ultrastructural tracing, we included molecular markers for 4-aminobutyrate (GABA), glutamate, glycine, taurine, glutamine, and the in vivo activity marker, 1-amino-4-guanidobutane. This enabled us to distinguish GABAergic and glycinergic amacrine cells; to identify ON bipolar cells coupled to glycinergic cells; and to discriminate different kinds of bipolar, amacrine, and ganglion cells based on their molecular signatures and activity. The data set was explored and annotated with Viking, our multiuser navigation tool. Annotations were exported to additional applications to render cells, visualize network graphs, and query the database.
Results: Exploration of RC1 showed that the 2 nm resolution readily recapitulated well known connections and revealed several new features of retinal organization: (1) The well known AII amacrine cell pathway displayed more complexity than previously reported, with no less than 17 distinct signaling modes, including ribbon synapse inputs from OFF bipolar cells, wide-field ON cone bipolar cells and rod bipolar cells, and extensive input from cone-pathway amacrine cells. (2) The axons of most cone bipolar cells formed a distinct signal integration compartment, with ON cone bipolar cell axonal synapses targeting diverse cell types. Both ON and OFF bipolar cells receive axonal veto synapses. (3) Chains of conventional synapses were very common, with intercalated glycinergic-GABAergic chains and very long chains associated with starburst amacrine cells. Glycinergic amacrine cells clearly play a major role in ON-OFF crossover inhibition. (4) Molecular and excitation mapping clearly segregates ultrastructurally defined bipolar cell groups into different response clusters. (5) Finally, low-resolution electron or optical imaging cannot reliably map synaptic connections by process geometry, as adjacency without synaptic contact is abundant in the retina. Only direct visualization of synapses and gap junctions suffices.
Conclusions: Connectome assembly and analysis using conventional transmission electron microscopy is now practical for network discovery. Our surveys of volume RC1 demonstrate that previously studied systems such as the AII amacrine cell network involve more network motifs than previously known. The AII network, primarily considered a scotopic pathway, clearly derives ribbon synapse input from photopic ON and OFF cone bipolar cell networks and extensive photopic GABAergic amacrine cell inputs. Further, bipolar cells show extensive inputs and outputs along their axons, similar to multistratified nonmammalian bipolar cells. Physiologic evidence of significant ON-OFF channel crossover is strongly supported by our anatomic data, showing alternating glycine-to-GABA paths. Long chains of amacrine cell networks likely arise from homocellular GABAergic synapses between starburst amacrine cells. Deeper analysis of RC1 offers the opportunity for more complete descriptions of specific networks.
Keywords: neuroscience, retina, vision, blindness, visus, crcns
2009
J.R. Anderson, B.W. Jones, J.-H. Yang, M.V. Shaw, C.B. Watt, P. Koshevoy, J. Spaltenstein, E. Jurrus, Kannan U.V., R.T. Whitaker, D. Mastronarde, T. Tasdizen, R.E. Marc.
“A Computational Framework for Ultrastructural Mapping of Neural Circuitry,” In PLoS Biology, Vol. 7, No. 3, pp. e74. 2009.
PubMed ID: 19855814
J. Anderson, B. Jones, J. Yang, M. Shaw, C. Watt, P. Koshevoy, J. Spaltenstein, E. Jurrus, Kannan U.V., R.T. Whitaker, D. Mastronarde, T. Tasdizen, R. Marc.
“Ultra Structural Mapping of Neural Circuitry: A Computational Framework,” In IEEE International Symposium on Biomedical Engineering (ISBI 2009), pp. 1135--1137. 2009.
DOI: 10.1109/ISBI.2009.5193257
Complete mapping of neuronal networks requires data acquisition at synaptic resolution with canonical coverage of tissues and robust neuronal classification. Transmission electron microscopy (TEM) remains the optimal tool for network mapping. However, capturing high resolution, large, serial section TEM (ssTEM) image volumes is complicated by the need to precisely mosaic distorted image tiles and subsequently register distorted mosaics. Moreover, most cell or tissue class markers are not optimized for TEM imaging. We present a complete framework for neuronal reconstruction at ultrastructural resolution, allowing the elucidation of complete neuronal circuits. This workflow combines TEM-compliant small molecule profiling with automated image tile mosaicking, automated slice-to-slice image registration and terabyte-scale image browsing for volume annotation. Networks that previously would require decades of assembly can now be completed in months, enabling large-scale connectivity analyses of both new and legacy data. Additionally, these approaches can be extended to other tissue or biological network systems.
Keywords: crcns, neural circuitry
E. Jurrus, M. Hardy, T. Tasdizen, P.T. Fletcher, P. Koshevoy, C.-B. Chien, W. Denk, R.T. Whitaker.
“Axon Tracking in Serial Block-Face Scanning Electron Microscopy,” In Medical Image Analysis (MEDIA), Vol. 13, No. 1, Elsevier, pp. 180--188. February, 2009.
PubMed ID: 18617436
Kannan U.V., A.R.C. Paiva, E. Jurrus, T. Tasdizen.
“Automatic Markup of Neural Cell Membranes Using Boosted Decision Stumps,” In Proceedings of the IEEE International Symposium on Biomedical Engineering (ISBI 2009), Boston, MA, pp. 1039--1042. 2009.
DOI: 10.1109/ISBI.2009.5193233
To better understand the central nervous system, neurobiologists need to reconstruct the underlying neural circuitry from electron microscopy images. One of the necessary tasks is to segment the individual neurons. For this purpose, we propose a supervised learning approach to detect the cell membranes. The classifier was trained using AdaBoost, on local and context features. The features were selected to highlight the line characteristics of cell membranes. It is shown that using features from context positions allows for more information to be utilized in the classification. Together with the nonlinear discrimination ability of the AdaBoost classifier, this results in clearly noticeable improvements over previously used methods.
Keywords: crcns, neural networks
Kannan UV, M. Kim, D. Gerszewski, J.R. Anderson, M. Hall.
“Assembling Large Mosaics of Electron Microscope Images using GPU,” In Proceedings of the 2009 Symposium on Application Accelerators in High Performance Computing (SAAHPC'09), 2009.
DOI: 10.1.1.163.213
Understanding the neural circuitry of the retina requires us to map the connectivity of individual neurons in large neuronal tissue sections and analyze signal communication across processes from the electron microscopy images. One of the major bottlenecks in the critical path is the image mosaicing process where 2D slices are assembled from scanned microscopy image tiles. The problem of assembling the tiles is computationally non-trivial because of distortion of the specimen in the electron microscope due to heat and overlap between the scanned tiles. The complexity of the calculation arises from the massive size of the dataset and mathematical calculations required to calculate value of each pixel of the mosaic. We propose to use texture memory lookups to speedup the access to image tiles and data parallel computing enabled by the GPUs to accelerate this process. The proposed method results in noticeable improvements in speed of computation compared to other methods. Index Terms—Serial-section TEM, image mosaicing, GPGPU, CUDA, texture.
Keywords: crcns, electron microscopy, mosaics, retina, eye
2008
E. Jurrus, R.T. Whitaker, B. Jones, R. Marc, T. Tasdizen.
“An Optimal-Path Approach for Neural Circuit Reconstruction,” In Proceedings of the 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, pp. 1609--1612. 2008.
PubMed ID: 19172170
2007
S. Gerber, T. Tasdizen, R.T. Whitaker.
“Robust Non-linear Dimensionality Reduction using Successive 1-Dimensional Laplacian Eigenmaps,” In Proceedings of the 2007 International Conference on Machine Learning (ICML), pp. 281--288. 2007.
B.W. Jones, R.E. Marc, C.B. Watt, K. Kinardi, D. DeMill, J.H. Yang, T. Tasdizen, P. Koshevoy, E. Jurrus, R.T. Whitaker.
“Structure and Function of Microneuromas in Retinal Remodeling,” In The Association for Research in Vision and Ophthalmology (ARVO) Conference, Note: (abstract), 2007.
P.A. Koshevoy, T. Tasdizen, R.T. Whitaker.
“Automatic Assembly of TEM Mosaics and Mosaic Stacks Using Phase Correlation,” SCI Institute Technical Report, No. UUSCI-2007-004, University of Utah, 2007.
2006
S.P. Awate, T. Tasdizen, R.T. Whitaker.
“Unsupervised Texture Segmentation with Nonparametric Neighborhood Statistics,” In Proceedings of The European Conference on Computer Vision (ECCV) 2006 Springer, Lecture Notes in Computer Science, pp. 494--507. 2006.
E. Jurrus, T. Tasdizen, P. Koshevoy, P.T. Fletcher, M. Hardy, C-B. Chien, W. Denk, R.T. Whitaker.
“Axon Tracking in Serial Block-Face Scanning Electron Microscopy,” In Workshop on Microscopic Image Analysis with Applications in Biology, MICCAI, October, 2006.
P.A. Koshevoy, T. Tasdizen, R.T. Whitaker.
“Implementation of an Automatic Slice-to-Slice Registration Tool,” SCI Institute Technical Report, No. UUSCI-2006-018, University of Utah, 2006.
P.A. Koshevoy, T. Tasdizen, R.T. Whitaker.
“Implementation of an Automatic Image Registration Tool,” SCI Institute Technical Report, No. UUSCI-2006-020, University of Utah, 2006.
P. Koshevoy, T. Tasdizen, R.T. Whitaker, B. Jones, R. Marc.
“Assembly of Large Three-Dimensional Volumes from Serial-Section Transmission Electron Microscopy,” In Proceedings of 2006 MICCAI Workshop on Microscopic Image Analysis with Applications in Biology, October, 2006.
2005
T. Tasdizen, R.T. Whitaker, R. Marc, B. Jones.
“Enhancement of Cell Boundaries in Transmission Micropscopy Images,” In IEEE International Conference on Image Processing, Vol. 2, pp. 129--132. 2005.
T. Tasdizen, R.T. Whitaker, R. Marc, B. Jones.
“Automatic Correction of Non-uniform Illumination in Transmission Electron Microscopy Images,” SCI Institute Technical Report, No. UUSCI-2005-008, University of Utah, 2005.
