Dissertation Defense
Defense

Defense Public Questioning
back to top
|
Specific Aims
The goal of my research is to create subject specific computer models of electrical activity in the heart that arise during conditions of compromised blood flow (myocardial ischemia). To accomplish this goal I will carry out the following aims:
-
Generate, validate, and compare image-based ischemia simulations against tradition modeling paradigms
I aim to accurately reconstruct, through computer simulation, the epicardial ST potentials generated by experimentally induced myocardial ischemia and compare both simulated and experimental results to those generated by traditional simulation methods. I will construct subject-specific models of ischemia by imposing experimentally derived ischemic zone geometries into image-based cardiac volumes—extracted from animal experiments. Epicardial recordings of corresponding animal experiments will be used to validate the model and provide a basis for comparison between subject-specific simulations and more traditional models.
-
Identify and optimize parametric ischemic zone geometries that accurately reflect epicardial potentials
I aim to develop parametric representations of ischemic sources capable of consistently, and accurately, replicating the epicardial ST potentials associated with acute myocardial ischemia. Using the above validated ischemia model, I will apply sophisticated, statistical methods to systematically manipulate ischemic zone parameters (e.g., location, size, shape, orientation) in order to identify optimal representations of the disease that reproduce epicardial ST potentials with a high degree of accuracy.
-
Assess the effect of variability in transmural fiber orientation on cardiac ST potentials during ischemia
I aim to implement a standard, rule-based fiber orientation scheme, within our image-based ischemia simula- tions, that allows for basic manipulation of fiber directionality parameters in order to assess the role that fiber architecture plays in ischemia. Within the applied fiber model, I will expose key, macroscopic fiber orientation parameters in order to smoothly alter cardiac fiber direction within the myocardium. By applying a series of systematic perturbations to these parameters, within human physiological ranges, I will generate a representative subset of ischemia models and determine the uncertainty in simulation outcomes associated with fiber orientation angle.
back to top
|
Background
Myocardial ischemia is a condition that results when the metabolic demand in a cardiac
region exceeds the available local supply of oxygen and nutrients. Early detection of myocardial
ischemia is necessary to direct clinical interventions, to evaluate their effectiveness, and to
monitor patients under conditions that may induce ischemia (e.g., stress testing or surgery).
However the ECG markers of ischemia are ambiguous and often inconclusive, resulting in diagnostic error rates of over 30% and unnecessary costs of over $5 billion annually in the US. Sources of error in clinical
detection are likely due to insufficient knowledge of the electrical consequences of ischemia
within the heart as well as variations in individual patient anatomy or electrode placement. Only
recently have researchers, with the aid of computational models, been able to carry out highparameter
analyses of such sources of uncertainty. Unfortunately, even these models are often
based on assumptions of ischemic zone location, geometry, and growth that are not producible in experimental settings; thus, they ignore
inter-subject variation and physiological ischemic zone formation.
I am contributing to the development of a simulation approach that supports subject specific
modeling and simulation of ischemia by creating customized geometric models based on detailed
imaging of the heart and ischemic regions. Such models, based on animal studies, will allow the complete evaluation of all relevant
parameters in creating accurate predictions of cardiac surface potentials resulting from episodes of acute, myocardial ischemia.
back to top
|
Methods
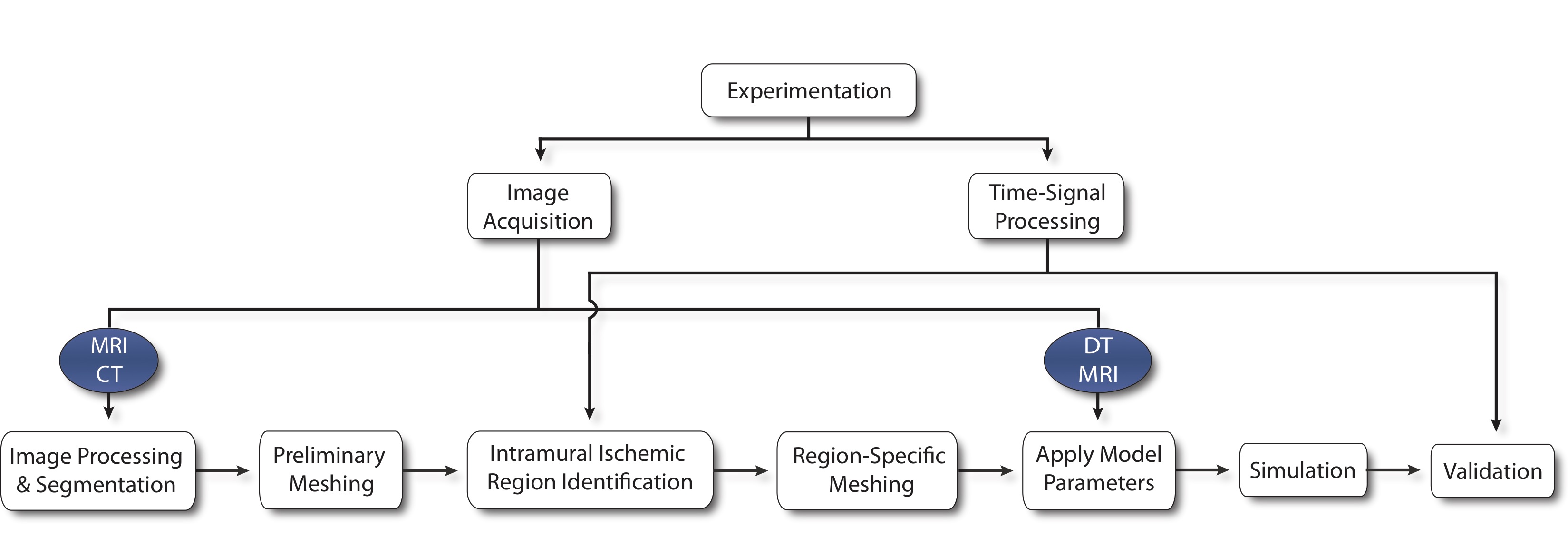
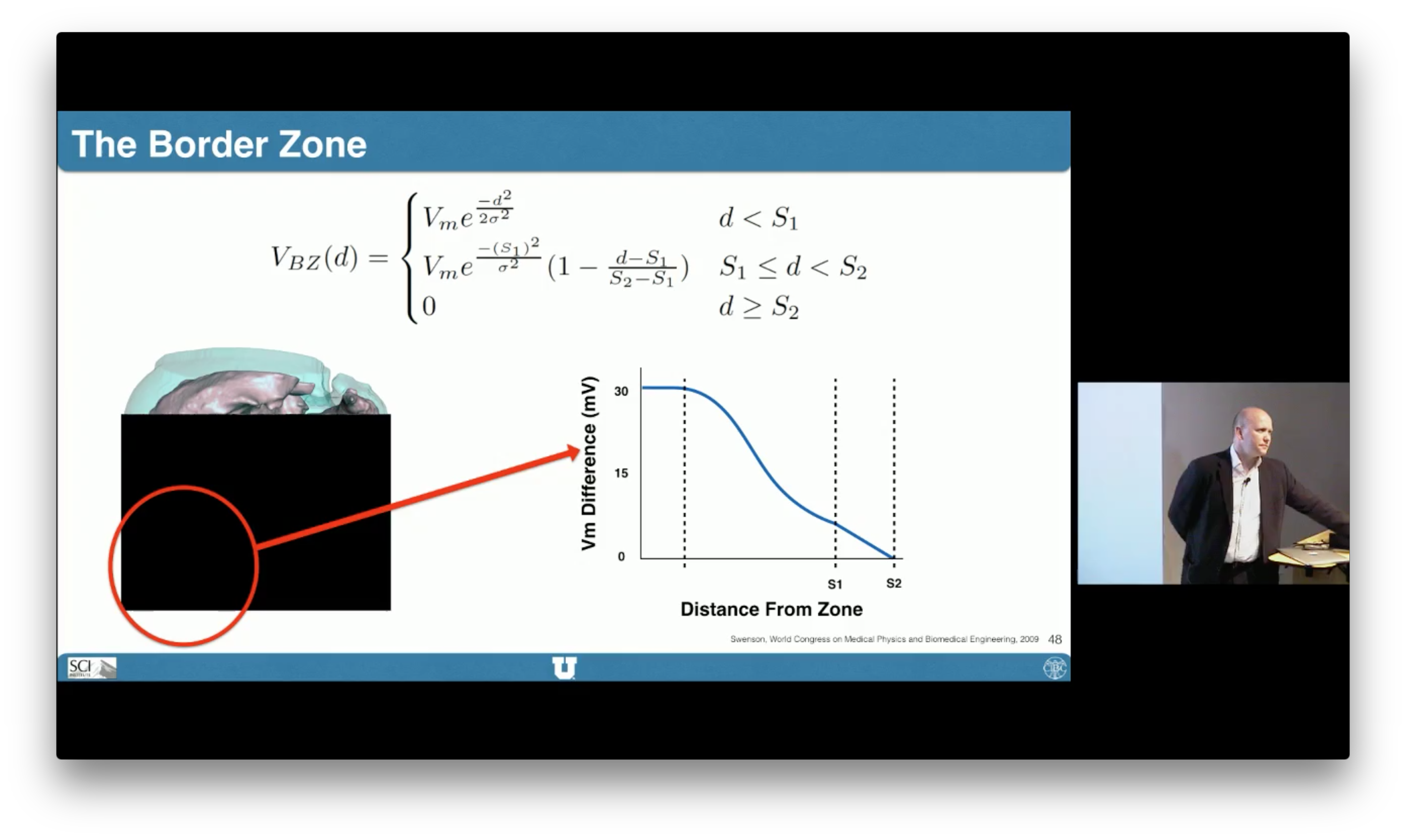
Simulation Pipeline
We begin by capturing subject-specific electrical recordings from an animal model of induced ischemia. Post experiment heart scans are processed to obtain both geometric and parametric data. Images are labeled and meshed to produce a geometric representation of the heart on which experimentally derived, transmural data can be mapped to identify ischemic regions. With ischemic zones defined, a new mesh is required to incorporate surface conforming ischemic regions within the tissue before fiber direction, conductivity, and initial conditions are applied. A finite element, passive current flow, bidomain simulation is used to generate extracellular potentials throughout the cardiac tissue that are validated against epicardial recordings previously extracted from the original experimental model.
For a more detailed description of methods used in my research, feel free to contact me or access my papers in the Publications tab.
back to top
|


