Simulation of Deep Brain Stimulation
Collaborating Investigator: Christopher R. Butson, Ph.D., Medical College of WisconsinDr. Butson is now a SCI Faculty member and Co-Investigator with CIBC.
Parkinson's Disease (PD) is a significant health problem for the aging population of the US. Despite the success of levodopa-based treatment for PD, many patients develop disabling motor side effects over time. One alternative for these patients is deep brain stimulation (DBS), a therapy in which a neurostimulation system is implanted in the brain during stereotactic surgery. This treatment has been FDA approved for approximately 10 years and its effectiveness in treating the motor symptoms of PD has been well established. However, DBS is not without its own side effects profile. Namely, it is heavily dependent on the intuitive skill of the clinician and no atlas exists for clinicians to share therapeutic or side effect outcomes. In this project, we propose to integrate these methods into a knowledge base that is viewable as an interactive, 3D atlas and to use this framework to examine neuropsychological outcomes in DBS patients with PD. The central hypothesis is that cognitive and psychological outcomes in these patients are correlated with stimulation-induced activation of specific brain regions. Therefore, one long-term objective is to better manage such outcomes by using the knowledge base created in this study to prescribe stimulation protocols that avoid activating these regions. Another potential result of this work is the ability to predict neuropsychological outcomes in DBS patients, which would help alleviate some of the long-term treatment costs this group experiences. To perform the proposed research we must solve two general computing problems: how to effectively generate large, complex, multiresolution, finite element models, and how to accurately visualize the model results. We anticipate that this study will generate a knowledge-base of clinical outcomes to be correlated with stimulation-induced activation of particular brain regions. The fundamental goal of this project is to characterize neuropsychological outcomes in PD patients who are receiving STN DBS. In order to create the greatest potential benefit with the simplest study design, we propose to use information gathered under existing standards of care for patient evaluation. Specifically, we propose to use pre- and postoperative imaging along with pre- and postoperative neuropsychological evaluation results. The novel component of this work is the integration of state-of-the-art clinical and research methods to improve the health of PD patients by characterizing neuropsychological outcomes.
We recently published results from a clinical study to assess the feasibility and utility of ImageVis3D Mobile for the selection of DBS settings for STN DBS cite1. We used the CIBC visualization system ImageVis3D Mobile to provide models to movement disorder clinicians and asked them to use the software to determine which of the four DBS electrode contacts they would select for therapy, and what stimulation settings they would choose. We compared the stimulation protocol chosen from the software versus the stimulation protocol that was chosen via clinical practice (independently of the study). We also compared the amount of time required to reach these settings using the software versus the time required through standard practice. We found that the stimulation settings chosen using ImageVis3D Mobile were similar to those used in standard care, but they were selected in drastically less time.
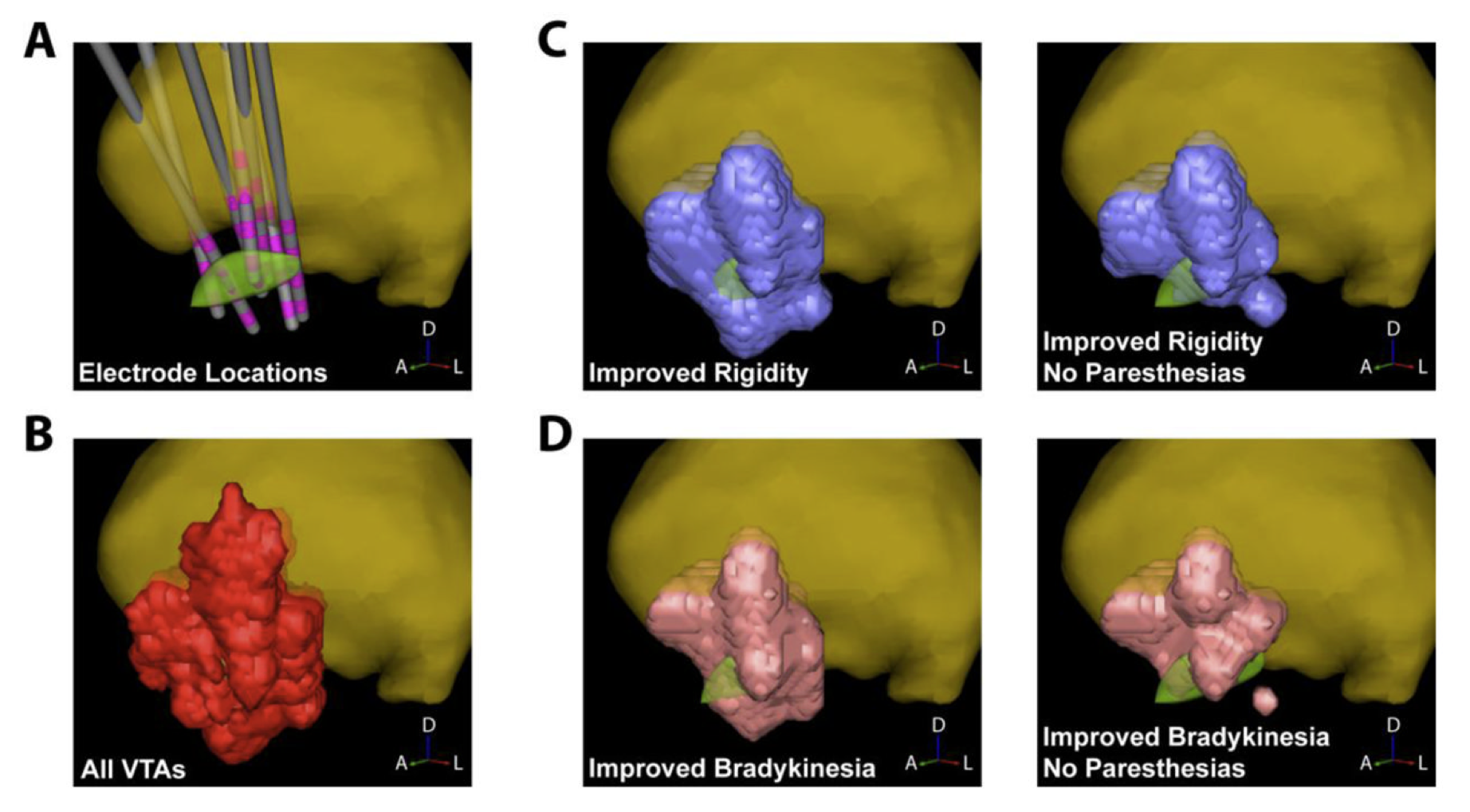
We are now planning the next phase of this project in which we will prospectively evaluate our platform. During this study, patients will be randomized to one of two approaches for postoperative selection of DBS settings: standard care and patient-specific models of DBS on ImageVis3D Mobile on an iPad. The study will be conducted in two phases. During the first phase we will evaluate whether clinician performance is improved, specifically the amount of time spent on DBS programming compared to standard care. During the second phase we will use the computational models and clinical outcomes from the first phase to build a probabilistic stimulation atlas (PSA) of effective and ineffective stimulation locations2. Clinical outcomes from this publication are shown in the Figure.
Targeting information from the PSA will be incorporated into the ImageVis3D Mobile patient models, and we will measure differences in therapeutic effectiveness and patient-reported quality of life for those patients compared to standard care. Based on our preliminary data we hypothesize that the use of interactive patient models will improve patient outcomes and clinician performance. Hence, this study attempts to quantify and compare the effects of DBS in a way that would not be possible without specialized computing tools. In this case the use of such tools has allowed us to study an informatics problem that is unique to neuromodulation therapies such as DBS, namely that its effects are acutely sensitive to where and how stimulation is delivered.
References:
1. C. Butson, G. Tamm, S. Jain, T. Fogal, and Jens Krueger. Evaluation of interactive visualization on mobile computing platforms for selection of deep brain stimulation parameters. IEEE Transactions on Visualization and Computer Graphics, 2012.
2. McIntyre C.C. Butson CR, Cooper SE, Henderson JM, Wolgamuth B. Probabilistic analysis of activation volumes generated during deep brain stimulation. Neuroimage, 1(54):2096–104, 2011.