Simulation of Cardiac Defibrillation
Collaborating Investigators:
- John Triedman, MD, Children's Hospital Boston
- Matt Jolley, MD, Stanford University
- Tom Pilcher, University of Utah
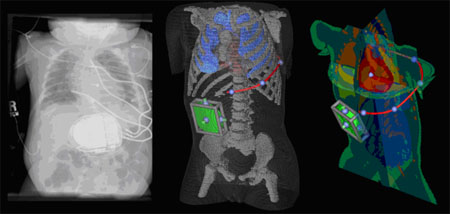
The purpose of the implantable cardioverter-defibrillator (ICD) is to monitor the heart rhythm of patients at risk and to intervene if necessary with an escalating series of artificial pacing that leads eventually to an electric shock capable of depolarizing most or all of the heart. Such therapy represents not a cure but an emergency precaution that studies have shown can extend the lives of patients who have a specific risk of ventricular tachycardias and fibrillation. This therapy has become so successful that in the US alone over 10,000 ICDs are implanted each month.
While many aspects of ICD use have seen impressive technical improvements, especially the sensing of arrhythmias and the recent additional of pacing protocols sometimes capable of restoring normal rhythm without the discomfort of a full shock, electrode placement has been largely static. There is a more or less standard placement paradigm that is effective
enough that there is little incentive for optimization; instead clinicians focus on reducing the time required and the fluoroscopy burden of the implantation operation. The situation in pediatric applications has been quite different, however, in part because of the small size of the patients and also because of the frequent presence of congenital anatomical anomalies that make the use of standard placement problematic. To respond to this need, we have carried out a longstanding DBP collaboration with Drs. John Triedman and Matt Jolley at the Childrens' Hospital Boston and, more recently, with Dr. Thomas Pilcher of the Primary Childrens' Hospital at the University of Utah.
Over the time of the collaboration, the CIBC has developed a complete simulation suite that allows for the creation of patient-specific torso models from CT or MRI scans, the interactive placement of the device and multiple electrodes within this model, and the simulation of the resulting defibrillation shock. Under standard clinical assumptions, it is even possible to predict the success of the shock.
The second area of progress has been with Dr. Pilcher in his studies of the effect of remnant metal, e.g. metal rods from spinal surgery or abandoned electrode wires from previous devices, on the defibrillation thresholds, ie the shock strength required for successful defibrillation. The results of these studies have led to a conference presentation5 and a manuscript is in final preparation.
Based on the maturity of the project and the relatively complete pipeline developed for this application, we intend to complete this DBP, according to the Collaborator Life Cycle plan within the CIBC. We have presented this proposal to the External Advisory Board, again as outlined in the Life Cycle strategy, and they have agreed to this plan. We will make all reasonable efforts to maintain the capability of the SCIRun networks and all other elements of the defibrillation pipeline even as we revise and update the software. This application will remain one of the test problems that we will use for validating new versions of the software and also for education and teaching purposes at workshops and training events.
References:
J.D. Tate, J.G. Stinstra, T. Pilcher, and R.S. MacLeod. Measuring implantable cardioverter defibrillators (ICDs) during implantation surgery: Verification of a simulation. In Computers in Cardiology, 2009, pages 473–476, 2009.J.D. Tate, J.G. Stinstra, T. Pilcher, and R.S. MacLeod. Implantable cardioverter defibrillator predictive simulation validation. In Computing in Cardiology, 2010, pages 853–856, 2010.
J.D. Tate, J.G. Stinstra, T. Pilcher, A. Poursaid, E.V. Saarel, and R.S. MacLeod. Simulating defibrillation: Verification using defibrillation thresholds and surface recordings. In Proceedings of Heart Rhythm Society, volume 8(5), page S396. Heart Rhythm Society, 2011
J. Tate, J. Stinstra, T. Pilcher, A. Poursaid, E. Saarel, and R. MacLeod. Measuring defibrillator surface potentials for simulation verification. Proceedings of the IEEE Engineering in Medicine and Biology Society 33rd Annual International Conference, 2011:239–242, 2011.
T.A. Pilcher, J.D. Tate, J.S. Stinstra, E.V. Saarel, A.E. Poursaid, M.D. Puchalski, and R.S. MacLeod. Effect of spinal fixation rods and abandoned epicardial patches on defibrillation thresholds predicted from computational simulation. In Proceedings of Heart Rhythm Society, volume 8(5), page S326, 2011.
