
Robert S. MacLeod - Bruno Taccardi - Robert L. Lux

The paper describes experimental studies of the effect of thorax conductivity on both torso and epicardial electric potentials. While considerable literature exists regarding the effect of tissue inhomogeneity on the transfer of heart electrical activity to the torso surface, very little has been written about whether or not the heart itself changes with altered thorax conductivity. We suspended an isolated, perfused dog heart in an electrolytic tank shaped like a human torso and recorded epicardial and torso potentials while we varied a) global conductivity of the electrolyte, or b) local conductivity by introducing insulators into the tank. Our results suggest that while the general topography of isopotential maps remains stable, changes in either global or local thoracic conductivity result in changes in the amplitudes of potentials on the epicardial surface. The implications of this work are important especially to forward and inverse solution modeling since it calls into doubt the implicit assumption that epicardial potentials are invariant to changes in the volume conductor.
While it is a fact that the conductivity of the thorax affects the manner in which cardiac bioelectricity manifests itself on the body surface (the electrocardiogram, or ECG), there remain many questions regarding the nature, degree, and both clinical and theoretical importance of this effect. The approaches to this problem have been varied, from computer models [1,2,3,4,5], to realistically shaped physical, electrolytic torso-tank models [6,7,8]. Despite over thirty years of effort, there is no consensus as to which of the inhomogeneities found in the thorax are significant, nor is it clear how best to include their effects into either mathematical models or clinical practice.
A question which has not been dealt with extensively in the past is whether or not the electrical conductivity of the thorax has any effect on the heart itself. The classical representation of the heart as one or more dipole vectors is inherently insensitive to the volume conductor which contains the heart. The more realistic representation of the heart's activity by the electric potential on an enclosing surface, while physically correct, describes the heart as what electrical engineers would call a ``voltage supply'', that is a perfect source capable of supplying enough current to maintain a specified voltage, irrespective of the ``load''. The currently most successful solutions to the forward and inverse problems in electrocardiography are based on such surface-based source descriptions of the heart as a voltage source.
When a forward solution model is used to predict torso potentials, the source is considered invariant. In modeling studies of the effect of thoracic inhomogeneities on the forward (and thus the inverse) solution, the approach has typically been to assign measured source potentials to the epicardium, and then compute torso potentials under different thorax conductivities. Differences in the torso potentials are assumed to be exclusively the result of changes in thoracic conductivity [3,5]. There is no allowance for the possibility that changing the thorax conductivity might alter the source potentials.
From a clinical perspective, the role of variations in thorax conductivity is known in principle, reducing ECG voltages when fluid builds up in the pericardium, for example. However, should both the thoracic conductivity and the heart itself be changing concomitantly, the sum of both effects must contribute to the ECG, thus weakening its diagnostic and monitoring utility. If, in addition, the heart potentials were to change because of altered thoracic conductivities, the ECG would be further distorted by a third component.
The suggestion that the nature of the volume in which the heart finds itself could affect the electric potentials on the heart surface came from experiments performed at this institute with an isolated whole heart preparation, placed in an electrolytic tank [9]. While results indicated that isochrone maps, and hence activation sequences, were not affected by removing the heart from the tank, potential amplitudes did change. Hence in the study described here, we set out to vary the conductivity of a realistically shaped electrolytic tank in which an isolated dog heart was suspended, and observe the changes in both torso tank, and epicardial electric potentials.
Analysis of the data consisted of first interactive visualization of the potential distributions, displayed on the digitized surfaces for both the heart and torso tank using a Unix graphics workstation and software developed specifically for this purpose [11]. In this way we could directly compare epicardial and torso potentials under different conductivity conditions. In order to quantify the differences induced by conductivity changes, we also extracted a number of characteristic signal parameters from each electrogram, including R-wave amplitude, S-wave amplitude, peak-to-peak variation during the QRS, T-wave amplitude, and the area under the signal over the QRS, ST, and QRST intervals (QRS, ST, and QRST ``integrals''). These values could then be displayed as ``iso-parameters'' on the digitized heart and tank surfaces as well as compared statistically between different beats to both quantify and localize the changes that altering the tank conductivity induced.
For this report, we present findings from just two specific experiments, one in which global conductivity was varied, the other in which two balloons were placed in the tank, one on either sides of the heart, or together at the back of the heart.
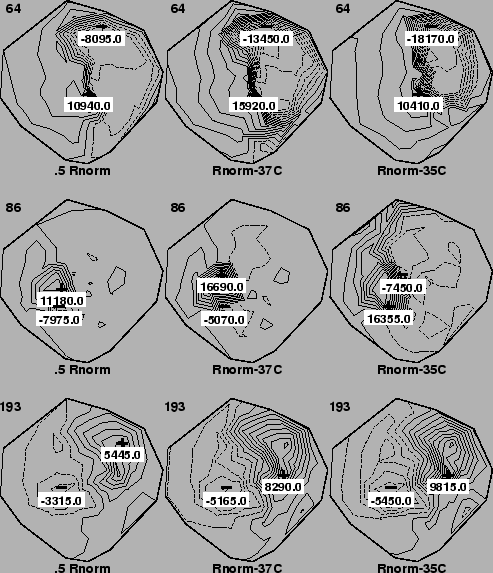
The two right-hand columns of Figure 1 contain epicardial
isopotential contour maps for our standard electrolyte (500 ![]() -cm at
room temperature). In the left-hand column are epicardial maps recorded in
an electrolyte adjusted to 250
-cm at
room temperature). In the left-hand column are epicardial maps recorded in
an electrolyte adjusted to 250 ![]() -cm (at room temperature). Temperature
during the experiment was 37o for the two left-hand columns and
35o for map in the right-hand column. Each row of maps represents
a different instant in time at which the recording was made.
-cm (at room temperature). Temperature
during the experiment was 37o for the two left-hand columns and
35o for map in the right-hand column. Each row of maps represents
a different instant in time at which the recording was made.
 |
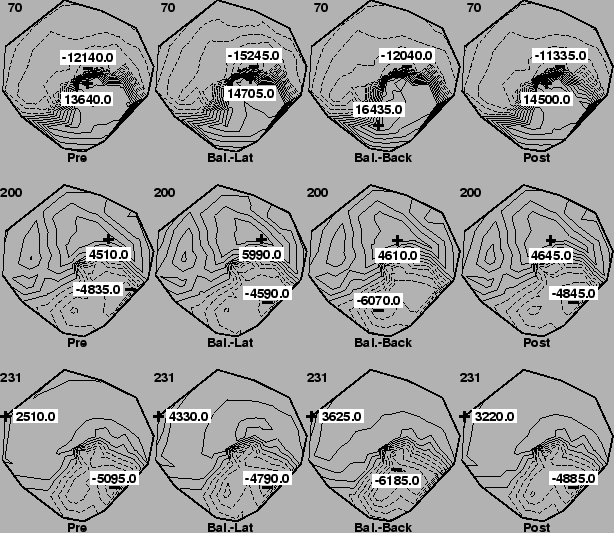
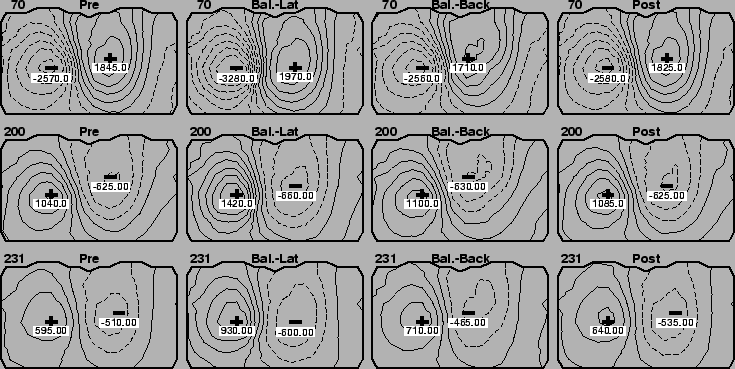
In Figures 2 and 3 are the isopotential maps for the epicardial and torso tank surfaces, respectively, during both the homogeneous condition and when the balloons were present. This particular case used right-ventricular pacing on the lateral right free wall of the heart and the times indicated in the upper left corners of each maps are in milliseconds after the stimulus.
 |
 |
A brief comparison of the maps across each row of the figures reveals that changes in thoracic conductivity do not appear to affect the pattern or topography of isopotential contours to a significant degree, which agrees with previous findings [9]. However, the magnitudes of the potentials do differ substantially from map to map, with the extrema in, for example, Figure 1, changing by 50% between normal and twice normal electrolyte conductivity. In fact, when we plotted the value of, for example, the R-wave amplitude for twice normal against normal conductivity for each electrode, the resulting line had a slope of about 0.7, suggesting an overall reduction in potential amplitude under conditions of increased conductivity.
The results from the balloon experiments were somewhat more subtle, but revealed regional changes in potential amplitudes when the balloons were present. In, for example, the first two rows of Figure 2, the extrema in the cases in which the balloons were located in back of the heart (third column) were enhanced over the case in the first and last columns in which no balloons were present. The changes were smaller than for the global conductivity change, but maxima nevertheless went from, for time instant 70, 13.6 mV before insertion to 14.7 mV with the balloons placed laterally, to 16.4 mV with balloons at the back of the heart back to 14.5 mV when the balloons were removed . Note also that the location of the this enhancement was at the back of the heart, near the location of the balloons.
A general finding was that when changes in potential amplitude occurred after balloons were inserted, these were usually regional in nature, and reflected a proximity to the location of the balloons relative to the heart. That is, when the balloons were located on both sides of the heart, changes in potentials, when they could be seen, were at the sides of the heart. The influence of the balloons appeared to be strongest when they were both located together at the back of the heart, perhaps a reflection of the increased volume concentration of the two balloons. Experiments in which we vary the size of the balloons, and more generally vary the shape and conductivity of inhomogeneous inserts in the tank, are planned.
The general nature of the effect of thoracic conductivity changes appears to be to reduce potential amplitudes when conductivity increases, and to increase potential amplitudes when conductivity is reduced. This fits well with the notion that increased conductivity near the heart provides enhanced paths for current leaving the heart to return to it, thus reducing the field strength near the torso.
Finally, it is interesting to note that changes in torso potentials can occur both with and without concomitant changes in epicardial potentials. The former case suggests that torso potentials simply follow the source without an additional effect from the inhomogeneous region in the thorax. In the latter case, we see proof that even under conditions of stable epicardial source potentials, thoracic inhomogeneities can alter the transfer of potential from the heart to the body surface.
The implications of these findings for modeling of the forward and inverse solutions are important. While a forward solution may be able to accurately predict torso potentials from epicardial sources, the solution must be improved by employing an accurate, inhomogeneous geometrical model. An estimate of the expected degree of improvement must await further study. However, our results suggest that such study cannot be carried out entirely in the modeling domain, since varying the torso model conductivity will require changing the source potentials, in a way as yet unpredictable by a model. Hence, the future of theoretical electrocardiography lies not within the supercomputers of tomorrow, but in a continued partnership between modeler and experimentalist.
We would like to thank Yonild Lian and Vince Lopez for expert technical assistance during the experiments. This research was support in part by awards from the Nora Eccles Treadwell Foundation and the Richard A. and Nora Eccles Harrison Fund for Cardiovascular Research.
This document was generated using the LaTeX2HTML translator Version 99.2beta6 (1.42)
Copyright © 1993, 1994, 1995, 1996,
Nikos Drakos,
Computer Based Learning Unit, University of Leeds.
Copyright © 1997, 1998, 1999,
Ross Moore,
Mathematics Department, Macquarie University, Sydney.
The command line arguments were:
latex2html -split 3 -no_white -link 3 -no_navigation -nomath -html_version 3.2,math paper
The translation was initiated by Rob MacLeod on 2000-08-18