SCI Publications
2016


J.L. Baker, J. Ryou, X.F. Wei, C.R. Butson, N.D. Schiff, K.P. Purpura.
“Robust modulation of arousal regulation, performance, and frontostriatal activity through central thalamic deep brain stimulation in healthy nonhuman primates,” In Journal of Neurophysiology, Vol. 116, No. 5, American Physiological Society, pp. 2383--2404. Aug, 2016.
DOI: 10.1152/jn.01129.2015
The central thalamus (CT) is a key component of the brain-wide network underlying arousal regulation and sensory-motor integration during wakefulness in the mammalian brain. Dysfunction of the CT, typically a result of severe brain injury (SBI), leads to long-lasting impairments in arousal regulation and subsequent deficits in cognition. Central thalamic deep brain stimulation (CT-DBS) is proposed as a therapy to reestablish and maintain arousal regulation to improve cognition in select SBI patients. However, a mechanistic understanding of CT-DBS and an optimal method of implementing this promising therapy are unknown. Here we demonstrate in two healthy nonhuman primates (NHPs), Macaca mulatta, that location-specific CT-DBS improves performance in visuomotor tasks and is associated with physiological effects consistent with enhancement of endogenous arousal. Specifically, CT-DBS within the lateral wing of the central lateral nucleus and the surrounding medial dorsal thalamic tegmental tract (DTTm) produces a rapid and robust modulation of performance and arousal, as measured by neuronal activity in the frontal cortex and striatum. Notably, the most robust and reliable behavioral and physiological responses resulted when we implemented a novel method of CT-DBS that orients and shapes the electric field within the DTTm using spatially separated DBS leads. Collectively, our results demonstrate that selective activation within the DTTm of the CT robustly regulates endogenous arousal and enhances cognitive performance in the intact NHP; these findings provide insights into the mechanism of CT-DBS and further support the development of CT-DBS as a therapy for reestablishing arousal regulation to support cognition in SBI patients.


W. Deeb, J. J. Giordano, P. J. Rossi, A. Y. Mogilner, A. Gunduz, J. W. Judy, B. T. Klassen, C. R. Butson, C. Van Horne, D. Deny, D. D. Dougherty, D. Rowell, G. A. Gerhardt, G. S. Smith, F. A. Ponce, H. C. Walker, H. M. Bronte-Stewart, H. S. Mayberg, H. J. Chizeck, J. Langevin, J. Volkmann, J. L. Ostrem, J. B. Shute, J. Jimenez-Shahed, K. D. Foote, A. W. Shukla, M. A. Rossi, M. Oh, M. Pourfar, P. B. Rosenberg, P. A. Silburn, C. de Hemptine, P. A. Starr, T. Denison, U. Akbar, W. M. Grill,, M. S. Okun.
“Proceedings of the Fourth Annual Deep Brain Stimulation Think Tank: A Review of Emerging Issues and Technologies,” In Frontiers in Integrative Neuroscience, Vol. 10, pp. 38. 2016.
ISSN: 1662-5145
DOI: 10.3389/fnint.2016.00038
This paper provides an overview of current progress in the technological advances and the use of deep brain stimulation (DBS) to treat neurological and neuropsychiatric disorders, as presented by participants of the Fourth Annual Deep Brain Stimulation Think Tank, which was convened in March 2016 in conjunction with the Center for Movement Disorders and Neurorestoration at the University of Florida, Gainesveille FL, USA. The Think Tank discussions first focused on policy and advocacy in DBS research and clinical practice, formation of registries, and issues involving the use of DBS in the treatment of Tourette Syndrome. Next, advances in the use of neuroimaging and electrochemical markers to enhance DBS specificity were addressed. Updates on ongoing use and developments of DBS for the treatment of Parkinson’s disease, essential tremor, Alzheimer’s disease, depression, post-traumatic stress disorder, obesity, addiction were presented, and progress toward innovation(s) in closed-loop applications were discussed. Each section of these proceedings provides updates and highlights of new information as presented at this year’s international Think Tank, with a view toward current and near future advancement of the field.


Y. Pathak, O. Salami, S. Baillet, Z. Li, C.R. Butson.
“Longitudinal Changes in Depressive Circuitry in Response to Neuromodulation Therapy,” In Frontiers in Neural Circuits, Vol. 10, rontiers Media SA, July, 2016.
DOI: 10.3389/fncir.2016.00050
BACKGROUND:
Major depressive disorder (MDD) is a public health problem worldwide. There is increasing interest in using non-invasive therapies such as repetitive transcranial magnetic stimulation (rTMS) to treat MDD. However, the changes induced by rTMS on neural circuits remain poorly characterized. The present study aims to test whether the brain regions previously targeted by deep brain stimulation (DBS) in the treatment of MDD respond to rTMS, and whether functional connectivity (FC) measures can predict clinical response.
METHODS:
rTMS (20 sessions) was administered to five MDD patients at the left-dorsolateral prefrontal cortex (L-DLPFC) over 4 weeks. Magnetoencephalography (MEG) recordings and Montgomery-Asberg depression rating scale (MADRS) assessments were acquired before, during and after treatment. Our primary measures, obtained with MEG source imaging, were changes in power spectral density (PSD) and changes in FC as measured using coherence.
RESULTS:
Of the five patients, four met the clinical response criterion (40% or greater decrease in MADRS) after 4 weeks of treatment. An increase in gamma power at the L-DLPFC was correlated with improvement in symptoms. We also found that increases in delta band connectivity between L-DLPFC/amygdala and L-DLPFC/pregenual anterior cingulate cortex (pACC), and decreases in gamma band connectivity between L-DLPFC/subgenual anterior cingulate cortex (sACC), were correlated with improvements in depressive symptoms.
CONCLUSIONS:
Our results suggest that non-invasive intervention techniques, such as rTMS, modulate the ongoing activity of depressive circuits targeted for DBS, and that MEG can capture these changes. Gamma oscillations may originate from GABA-mediated inhibition, which increases synchronization of large neuronal populations, possibly leading to increased long-range FC. We postulate that responses to rTMS could provide valuable insights into early evaluation of patient candidates for DBS surgery.
2015


T. Bregman, R. Reznikov, M. Diwan, R. Raymond, C. R.Butson, J. N.Nobrega, C. Hamani.
“Antidepressant-like Effects of Medial Forebrain Bundle Deep Brain Stimulation in Rats are not Associated With Accumbens Dopamine Release,” In Brain Stimulation, Vol. 8, No. 4, pp. 708--713. 2015.
BACKGROUND:
Medial forebrain bundle (MFB) deep brain stimulation (DBS) is currently being investigated in patients with treatment-resistant depression. Striking features of this therapy are the large number of patients who respond to treatment and the rapid nature of the antidepressant response.
OBJECTIVE:
To study antidepressant-like behavioral responses, changes in regional brain activity, and monoamine release in rats receiving MFB DBS.
METHODS:
Antidepressant-like effects of MFB stimulation at 100 μA, 90 μs and either 130 Hz or 20 Hz were characterized in the forced swim test (FST). Changes in the expression of the immediate early gene (IEG) zif268 were measured with in situ hybridization and used as an index of regional brain activity. Microdialysis was used to measure DBS-induced dopamine and serotonin release in the nucleus accumbens.
RESULTS:
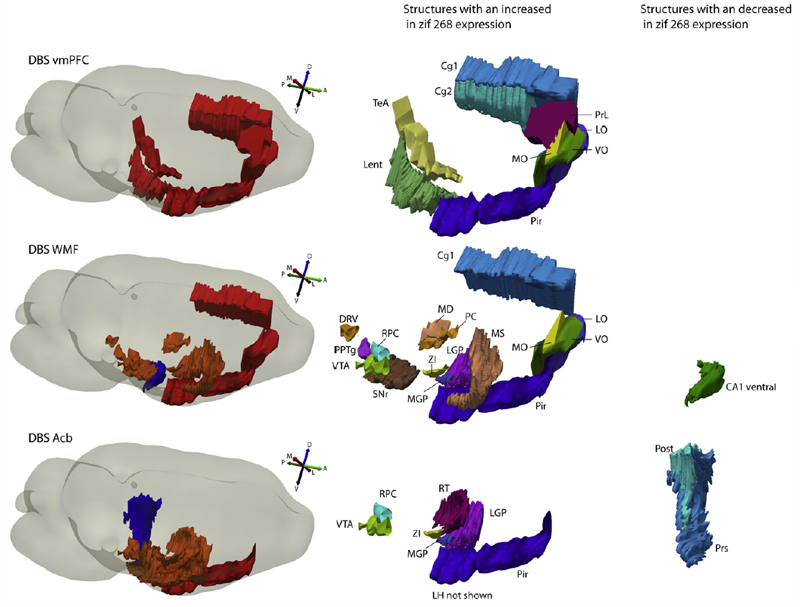
Stimulation at parameters that approximated those used in clinical practice, but not at lower frequencies, induced a significant antidepressant-like response in the FST. In animals receiving MFB DBS at high frequency, increases in zif268 expression were observed in the piriform cortex, prelimbic cortex, nucleus accumbens shell, anterior regions of the caudate/putamen and the ventral tegmental area. These structures are involved in the neurocircuitry of reward and are also connected to other brain areas via the MFB. At settings used during behavioral tests, stimulation did not induce either dopamine or serotonin release in the nucleus accumbens.
CONCLUSIONS:
These results suggest that MFB DBS induces an antidepressant-like effect in rats and recruits structures involved in the neurocircuitry of reward without affecting dopamine release in the nucleus accumbens.


C. R. Butson, C. C. McIntyre.
“The use of stimulation field models for deep brain stimulation programming,” In Brain Stimulation, Vol. 8, No. 5, Elsevier BV, pp. 976--978. September, 2015.
DOI: 10.1016/j.brs.2015.06.005


B. D. Goodwin, C. R. Butson.
“Subject-Specific Multiscale Modeling to Investigate Effects of Transcranial Magnetic Stimulation,” In Neuromodulation: Technology at the Neural Interface, Vol. 18, No. 8, Wiley-Blackwell, pp. 694--704. May, 2015.
DOI: 10.1111/ner.12296
OBJECTIVE:
Transcranial magnetic stimulation (TMS) is an effective intervention in noninvasive neuromodulation used to treat a number of neurophysiological disorders. Predicting the spatial extent to which neural tissue is affected by TMS remains a challenge. The goal of this study was to develop a computational model to predict specific locations of neural tissue that are activated during TMS. Using this approach, we assessed the effects of changing TMS coil orientation and waveform.
MATERIALS AND METHODS:
We integrated novel techniques to develop a subject-specific computational model, which contains three main components: 1) a figure-8 coil (Magstim, Magstim Company Limited, Carmarthenshire, UK); 2) an electromagnetic, time-dependent, nonhomogeneous, finite element model of the whole head; and 3) an adaptation of a previously published pyramidal cell neuron model. We then used our modeling approach to quantify the spatial extent of affected neural tissue for changes in TMS coil rotation and waveform.
RESULTS:
We found that our model shows more detailed predictions than previously published models, which underestimate the spatial extent of neural activation. Our results suggest that fortuitous sites of neural activation occur for all tested coil orientations. Additionally, our model predictions show that excitability of individual neural elements changes with a coil rotation of ±15°.
CONCLUSIONS:
Our results indicate that the extent of neuromodulation is more widespread than previous published models suggest. Additionally, both specific locations in cortex and the extent of stimulation in cortex depend on coil orientation to within ±15° at a minimum. Lastly, through computational means, we are able to provide insight into the effects of TMS at a cellular level, which is currently unachievable by imaging modalities.


A. Gunduz, H. Morita, P. J. Rossi, W. L. Allen, R. L. Alterman, H. Bronte-Stewart, C. R. Butson, D. Charles, S. Deckers, C. de Hemptinne, M. DeLong, D. Dougherty, J. Ellrich, K. D. Foote, J. Giordano, W. Goodman, B. D. Greenberg, D. Greene, R. Gross, J. W. Judy, E. Karst, A. Kent, B. Kopell, A. Lang, A. Lozano, C. Lungu, K. E. Lyons, A. Machado, H. Martens, C. McIntyre, H. Min, J. Neimat, J. Ostrem, S. Pannu, F. Ponce, N. Pouratian, D. Reymers, L. Schrock, S. Sheth, L. Shih, S. Stanslaski, G. K. Steinke, P. Stypulkowski, A. I. Tröster, L. Verhagen, H. Walker, M. S. Okun.
“Proceedings of the Second Annual Deep Brain Stimulation Think Tank: What's in the Pipeline,” In International Journal of Neuroscience, Vol. 125, No. 7, Taylor & Francis, pp. 475-485. 2015.
DOI: 10.3109/00207454.2014.999268
PubMed ID: 25526555
The proceedings of the 2nd Annual Deep Brain Stimulation Think Tank summarize the most contemporary clinical, electrophysiological, and computational work on DBS for the treatment of neurological and neuropsychiatric disease and represent the insights of a unique multidisciplinary ensemble of expert neurologists, neurosurgeons, neuropsychologists, psychiatrists, scientists, engineers and members of industry. Presentations and discussions covered a broad range of topics, including advocacy for DBS, improving clinical outcomes, innovations in computational models of DBS, understanding of the neurophysiology of Parkinson's disease (PD) and Tourette syndrome (TS) and evolving sensor and device technologies.
2014


M.S. Okun, S.S. Wu, S. Fayad, H. Ward, D. Bowers, C. Rosado, L. Bowen, C. Jacobson, C.R. Butson, K.D. Foote.
“Acute and Chronic Mood and Apathy Outcomes from a Randomized Study of Unilateral STN and GPi DBS,” In PLoS ONE, Vol. 9, No. 12, pp. e114140. December, 2014.
Objective: To study mood and behavioral effects of unilateral and staged bilateral subthalamic nucleus (STN) and globus pallidus internus (GPi) deep brain stimulation (DBS) for Parkinson's disease (PD).
Background: There are numerous reports of mood changes following DBS, however, most have focused on bilateral simultaneous STN implants with rapid and aggressive post-operative medication reduction.
Methods: A standardized evaluation was applied to a subset of patients undergoing STN and GPi DBS and who were also enrolled in the NIH COMPARE study. The Unified Parkinson Disease Rating Scale (UPDRS III), the Hamilton depression (HAM-D) and anxiety rating scales (HAM-A), the Yale-Brown obsessive-compulsive rating scale (YBOCS), the Apathy Scale (AS), and the Young mania rating scale (YMRS) were used. The scales were repeated at acute and chronic intervals. A post-operative strategy of non-aggressive medication reduction was employed.
Results: Thirty patients were randomized and underwent unilateral DBS (16 STN, 14 GPi). There were no baseline differences. The GPi group had a higher mean dopaminergic dosage at 1-year, however the between group difference in changes from baseline to 1-year was not significant. There were no differences between groups in mood and motor outcomes. When combining STN and GPi groups, the HAM-A scores worsened at 2-months, 4-months, 6-months and 1-year when compared with baseline; the HAM-D and YMRS scores worsened at 4-months, 6-months and 1-year; and the UPDRS Motor scores improved at 4-months and 1-year. Psychiatric diagnoses (DSM-IV) did not change. No between group differences were observed in the cohort of bilateral cases.
Conclusions: There were few changes in mood and behavior with STN or GPi DBS. The approach of staging STN or GPi DBS without aggressive medication reduction could be a viable option for managing PD surgical candidates. A study of bilateral DBS and of medication reduction will be required to better understand risks and benefits of a bilateral approach.


S.E. Cooper, K.G. Driesslein, A.M. Noecker, C.C. McIntyre, A.M. Machado, C.R. Butson.
“Anatomical targets associated with abrupt versus gradual washout of subthalamic deep brain stimulation effects on bradykinesia,” In PloS One, Vol. 9, No. 8, pp. e99663. January, 2014.
ISSN: 1932-6203
DOI: 10.1371/journal.pone.0099663
PubMed ID: 25098453
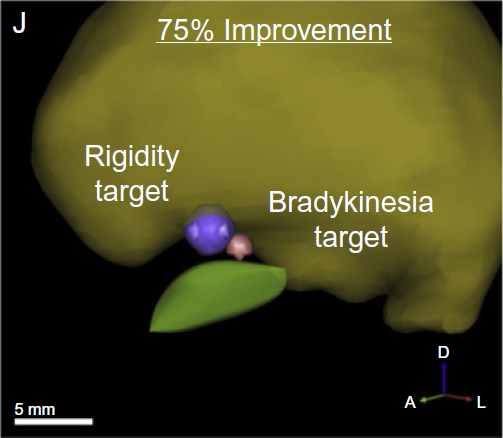
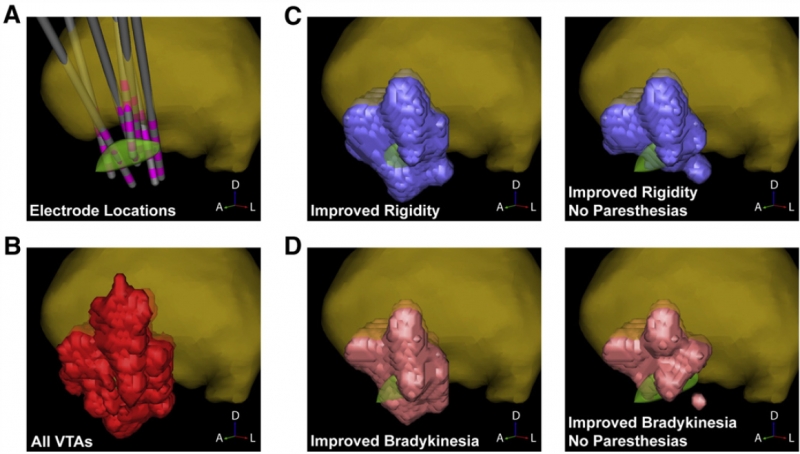
The subthalamic nucleus (STN) is a common anatomical target for deep brain stimulation (DBS) for the treatment of Parkinson's disease. However, the effects of stimulation may spread beyond the STN. Ongoing research aims to identify nearby anatomical structures where DBS-induced effects could be associated with therapeutic improvement or side effects. We previously found that DBS lead location determines the rate--abrupt vs. gradual--with which therapeutic effect washes out after stimulation is stopped. Those results suggested that electrical current spreads from the electrodes to two spatially distinct stimulation targets associated with different washout rates. In order to identify these targets we used computational models to predict the volumes of tissue activated during DBS in 14 Parkinson's patients from that study. We then coregistered each patient with a stereotaxic atlas and generated a probabilistic stimulation atlas to obtain a 3-dimensional representation of regions where stimulation was associated with abrupt vs. gradual washout. We found that the therapeutic effect which washed out gradually was associated with stimulation of the zona incerta and fields of Forel, whereas abruptly-disappearing therapeutic effect was associated with stimulation of STN itself. This supports the idea that multiple DBS targets exist and that current spread from one electrode may activate more than one of them in a given patient, producing a combination of effects which vary according to electrode location and stimulation settings.


C. Hamani, B.O. Amorim, A.L. Wheeler, M. Diwan, K. Driesslein, L. Covolan, C.R. Butson, J.N. Nobrega.
“Deep brain stimulation in rats: Different targets induce similar antidepressant-like effects but influence different circuits,” In Neurobiology of Disease, Vol. 71, Elsevier Inc., pp. 205--214. August, 2014.
ISSN: 1095-953X
DOI: 10.1016/j.nbd.2014.08.007
PubMed ID: 25131446
Recent studies in patients with treatment-resistant depression have shown similar results with the use of deep brain stimulation (DBS) in the subcallosal cingulate gyrus (SCG), ventral capsule/ventral striatum (VC/VS) and nucleus accumbens (Acb). As these brain regions are interconnected, one hypothesis is that by stimulating these targets one would just be influencing different relays in the same circuitry. We investigate behavioural, immediate early gene expression, and functional connectivity changes in rats given DBS in homologous regions, namely the ventromedial prefrontal cortex (vmPFC), white matter fibers of the frontal region (WMF) and nucleus accumbens. We found that DBS delivered to the vmPFC, Acb but not WMF induced significant antidepressant-like effects in the FST (31\%, 44\%, and 17\% reduction in immobility compared to controls). Despite these findings, stimulation applied to these three targets induced distinct patterns of regional activity and functional connectivity. While animals given vmPFC DBS had increased cortical zif268 expression, changes after Acb stimulation were primarily observed in subcortical structures. In animals receiving WMF DBS, both cortical and subcortical structures at a distance from the target were influenced by stimulation. In regards to functional connectivity, DBS in all targets decreased intercorrelations among cortical areas. This is in contrast to the clear differences observed in subcortical connectivity, which was reduced after vmPFC DBS but increased in rats receiving Acb or WMF stimulation. In conclusion, results from our study suggest that, despite similar antidepressant-like effects, stimulation of the vmPFC, WMF and Acb induce distinct changes in regional brain activity and functional connectivity.
Keywords: Anterior capsule, Connectivity, Deep brain stimulation, Depression, Nucleus accumbens, Prefrontal cortex


K.A. Nestor, J.D. Jones, C.R. Butson, T. Morishita, C.E. Jacobson, D.A. Peace, D. Chen, K.D. Foote, M.S. Okun.
“Coordinate-based lead location does not predict Parkinson's disease deep brain stimulation outcome,” In PloS One, Vol. 9, No. 4, pp. e93524. January, 2014.
ISSN: 1932-6203
DOI: 10.1371/journal.pone.0093524
PubMed ID: 24691109
BACKGROUND: Effective target regions for deep brain stimulation (DBS) in Parkinson's disease (PD) have been well characterized. We sought to study whether the measured Cartesian coordinates of an implanted DBS lead are predictive of motor outcome(s). We tested the hypothesis that the position and trajectory of the DBS lead relative to the mid-commissural point (MCP) are significant predictors of clinical outcomes. We expected that due to neuroanatomical variation among individuals, a simple measure of the position of the DBS lead relative to MCP (commonly used in clinical practice) may not be a reliable predictor of clinical outcomes when utilized alone.
METHODS: 55 PD subjects implanted with subthalamic nucleus (STN) DBS and 41 subjects implanted with globus pallidus internus (GPi) DBS were included. Lead locations in AC-PC space (x, y, z coordinates of the active contact and sagittal and coronal entry angles) measured on high-resolution CT-MRI fused images, and motor outcomes (Unified Parkinson's Disease Rating Scale) were analyzed to confirm or refute a correlation between coordinate-based lead locations and DBS motor outcomes.
RESULTS: Coordinate-based lead locations were not a significant predictor of change in UPDRS III motor scores when comparing pre- versus post-operative values. The only potentially significant individual predictor of change in UPDRS motor scores was the antero-posterior coordinate of the GPi lead (more anterior lead locations resulted in a worse outcome), but this was only a statistical trend (p<.082).
CONCLUSION: The results of the study showed that a simple measure of the position of the DBS lead relative to the MCP is not significantly correlated with PD motor outcomes, presumably because this method fails to account for individual neuroanatomical variability. However, there is broad agreement that motor outcomes depend strongly on lead location. The results suggest the need for more detailed identification of stimulation location relative to anatomical targets.
2013


K. Fakhar, E. Hastings, C.R. Butson, K.D. Foote, P. Zeilman, M.S. Okun.
“Management of deep brain stimulator battery failure: battery estimators, charge density, and importance of clinical symptoms,” In PloS One, Vol. 8, No. 3, pp. e58665. January, 2013.
ISSN: 1932-6203
DOI: 10.1371/journal.pone.0058665
PubMed ID: 23536810
We aimed in this investigation to study deep brain stimulation (DBS) battery drain with special attention directed toward patient symptoms prior to and following battery replacement.


N. Farah, A. Zoubi, S. Matar, L. Golan, A. Marom, C.R. Butson, I. Brosh, S. Shoham.
“Holographically patterned activation using photo-absorber induced neural-thermal stimulation,” In Journal of Neural Engineering, Vol. 10, No. 5, pp. 056004. October, 2013.
ISSN: 1741-2560
DOI: 10.1088/1741-2560/10/5/056004
Objective. Patterned photo-stimulation offers a promising path towards the effective control of distributed neuronal circuits. Here, we demonstrate the feasibility and governing principles of spatiotemporally patterned microscopic photo-absorber induced neural.thermal stimulation (PAINTS) based on light absorption by exogenous extracellular photo-absorbers. Approach. We projected holographic light patterns from a green continuous-wave (CW) or an IR femtosecond laser onto exogenous photo-absorbing particles dispersed in the vicinity of cultured rat cortical cells. Experimental results are compared to predictions of a temperature-rate model (where membrane currents follow I ∝ dT/dt). Main results. The induced microscopic photo-thermal transients have sub-millisecond thermal relaxation times and stimulate adjacent cells. PAINTS activation thresholds for different laser pulse durations (0.02 to 1 ms) follow the Lapicque strength-duration formula, but with different chronaxies and minimal threshold energy levels for the two excitation lasers (an order of magnitude lower for the IR system mporal selectivity.


Y. Pathak, B.H. Kopell, A. Szabo, C. Rainey, H. Harsch, C.R. Butson.
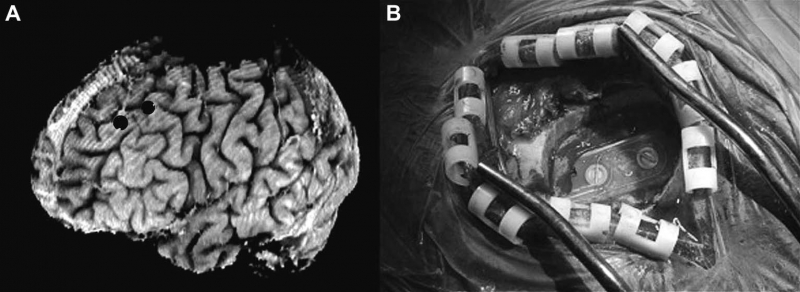
“The role of electrode location and stimulation polarity in patient response to cortical stimulation for major depressive disorder,” In Brain Stimulation, Vol. 6, No. 3, Elsevier Ltd., pp. 254--260. July, 2013.
ISSN: 1935-861X
DOI: 10.1016/j.brs.2012.07.001
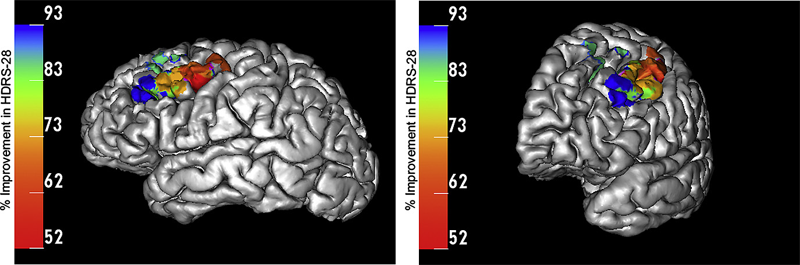
BACKGROUND: Major depressive disorder (MDD) is a neuropsychiatric condition that affects about one-sixth of the US population. Chronic epidural stimulation (EpCS) of the left dorsolateral prefrontal cortex (DLPFC) was recently evaluated as a treatment option for refractory MDD and was found to be effective during the open-label phase. However, two potential sources of variability in the study were differences in electrode position and the range of stimulation modes that were used in each patient. The objective of this study was to examine these factors in an effort to characterize successful EpCS therapy.
METHODS: Data were analyzed from eleven patients who received EpCS via a chronically implanted system. Estimates were generated of response probability as a function of duration of stimulation. The relative effectiveness of different stimulation modes was also evaluated. Lastly, a computational analysis of the pre- and post-operative imaging was performed to assess the effects of electrode location. The primary outcome measure was the change in Hamilton Depression Rating Scale (HDRS-28).
RESULTS: Significant improvement was observed in mixed mode stimulation (alternating cathodic and anodic) and continuous anodic stimulation (full power). The changes observed in HDRS-28 over time suggest that 20 weeks of stimulation are necessary to approach a 50\% response probability. Lastly, stimulation in the lateral and anterior regions of DLPFC was correlated with greatest degree of improvement.
CONCLUSIONS: A persistent problem in neuromodulation studies has been the selection of stimulation parameters and electrode location to provide optimal therapeutic response. The approach used in this paper suggests that insights can be gained by performing a detailed analysis of response while controlling for important details such as electrode location and stimulation settings.
Keywords: cortical stimulation


W.C. Stacey, S. Kellis, B. Greger, C.R. Butson, P.R. Patel, T. Assaf, T. Mihaylova, S. Glynn.
“Potential for unreliable interpretation of EEG recorded with microelectrodes,” In Epilepsia, May, 2013.
ISSN: 00139580
DOI: 10.1111/epi.12202
2012


C.R. Butson.
“Computational Models of Neuromodulation,” In Emerging Horizons in Neuromodulation, Edited by Elena Moro and Clement Hamani, Academic Press, pp. 5--22. 2012.
ISBN: 978012404706
As neuromodulation therapy has grown, so has the recognition that computational models can provide important insights into the design, operation, and clinical application of neurostimulation systems. Models of deep brain stimulation and spinal cord stimulation have advanced over recent decades from simple, stereotyped models to sophisticated patient-specific models that can incorporate many important details of the stimulation system and the attributes of individual subjects. Models have been used to make detailed predictions of the bioelectric fields produced during stimulation. These predictions have been used as a starting point for further analyses such as stimulation safety, neural response, neurostimulation system design, or clinical outcomes. This chapter provides a review of recent advances and anticipated future directions in computational modeling of neuromodulation.


W.C. Stacey, S. Kellis, P.R. Patel, B. Greger, C.R. Butson.
“Signal distortion from microelectrodes in clinical EEG acquisition systems,” In Journal of Neural Engineering, Vol. 9, No. 5, pp. 056007. October, 2012.
ISSN: 1741-2552
DOI: 10.1088/1741-2560/9/5/056007
PubMed ID: 22878608
Many centers are now using high-density microelectrodes during traditional intracranial electroencephalography (iEEG) both for research and clinical purposes. These microelectrodes are FDA-approved and integrate into clinical EEG acquisition systems. However, the electrical characteristics of these electrodes are poorly described and clinical systems were not designed to use them; thus, it is possible that this shift into clinical practice could have unintended consequences. In this study, we characterized the impedance of over 100 commercial macro- and microelectrodes using electrochemical impedance spectroscopy (EIS) to determine how electrode properties could affect signal acquisition and interpretation. The EIS data were combined with the published specifications of several commercial EEG systems to design digital filters that mimic the behavior of the electrodes and amplifiers. These filters were used to analyze simulated brain signals that contain a mixture of characteristic features commonly observed in iEEG. Each output was then processed with several common quantitative EEG measurements. Our results show that traditional macroelectrodes had low impedances and produced negligible distortion of the original signal. Brain tissue and electrical wiring also had negligible filtering effects. However, microelectrode impedances were much higher and more variable than the macroelectrodes. When connected to clinical amplifiers, higher impedance electrodes produced considerable distortion of the signal at low frequencies (
2011


C.R. Butson, S.E. Cooper, J.M. Henderson, B. Wolgamuth, C.C. McIntyre.
“Probabilistic Analysis of Activation Volumes Generated During Deep Brain Stimulation,” In NeuroImage, Vol. 54, pp. 2096--2104. 2011.
ISSN: 1095-9572
DOI: 10.1016/j.neuroimage.2010.10.059
PubMed ID: 20974269
Deep brain stimulation (DBS) is an established therapy for the treatment of Parkinson's disease (PD) and shows great promise for the treatment of several other disorders. However, while the clinical analysis of DBS has received great attention, a relative paucity of quantitative techniques exists to define the optimal surgical target and most effective stimulation protocol for a given disorder. In this study we describe a methodology that represents an evolutionary addition to the concept of a probabilistic brain atlas, which we call a probabilistic stimulation atlas (PSA). We outline steps to combine quantitative clinical outcome measures with advanced computational models of DBS to identify regions where stimulation-induced activation could provide the best therapeutic improvement on a per-symptom basis. While this methodology is relevant to any form of DBS, we present example results from subthalamic nucleus (STN) DBS for PD. We constructed patient-specific computer models of the volume of tissue activated (VTA) for 163 different stimulation parameter settings which were tested in six patients. We then assigned clinical outcome scores to each VTA and compiled all of the VTAs into a PSA to identify stimulation-induced activation targets that maximized therapeutic response with minimal side effects. The results suggest that selection of both electrode placement and clinical stimulation parameter settings could be tailored to the patient's primary symptoms using patient-specific models and PSAs.


C.R. Butson, I.O. Miller, R.A. Normann, G.A. Clark.
“Selective neural activation in a histologically derived model of peripheral nerve,” In Journal of Neural Engineering, Vol. 8, No. 3, pp. 036009. June, 2011.
ISSN: 1741-2552
DOI: 10.1088/1741-2560/8/3/036009
PubMed ID: 21478574
Functional electrical stimulation (FES) is a general term for therapeutic methods that use electrical stimulation to aid or replace lost ability. For FES systems that communicate with the nervous system, one critical component is the electrode interface through which the machine-body information transfer must occur. In this paper, we examine the influence of inhomogeneous tissue conductivities and positions of nodes of Ranvier on activation of myelinated axons for neuromuscular control as a function of electrode configuration. To evaluate these effects, we developed a high-resolution bioelectric model of a fascicle from a stained cross-section of cat sciatic nerve. The model was constructed by digitizing a fixed specimen of peripheral nerve, extruding the image along the axis of the nerve, and assigning each anatomical component to one of several different tissue types. Electrodes were represented by current sources in monopolar, transverse bipolar, and longitudinal bipolar configurations; neural activation was determined using coupled field-neuron simulations with myelinated axon cable models. We found that the use of an isotropic tissue medium overestimated neural activation thresholds compared with the use of physiologically based, inhomogeneous tissue medium, even after controlling for mean impedance levels. Additionally, the positions of the cathodic sources relative to the nodes of Ranvier had substantial effects on activation, and these effects were modulated by the electrode configuration. Our results indicate that physiologically based tissue properties cause considerable variability in the neural response, and the inclusion of these properties is an important component in accurately predicting activation. The results are used to suggest new electrode designs to enable selective stimulation of small diameter fibers.


B.H. Kopell, J. Halverson, C.R. Butson, M. Dickinson, J. Bobholz, H. Harsch, C. Rainey, D. Kondziolka, R. Howland, E. Eskandar, K.C. Evans, D.D. Dougherty.
“Epidural cortical stimulation of the left dorsolateral prefrontal cortex for refractory major depressive disorder,” In Neurosurgery, Vol. 69, No. 5, pp. 1015--1029. November, 2011.
ISSN: 1524-4040
DOI: 10.1227/NEU.0b013e318229cfcd
A significant number of patients with major depressive disorder are unresponsive to conventional therapies. For these patients, neuromodulation approaches are being investigated.